The Burden of Myelofibrosis
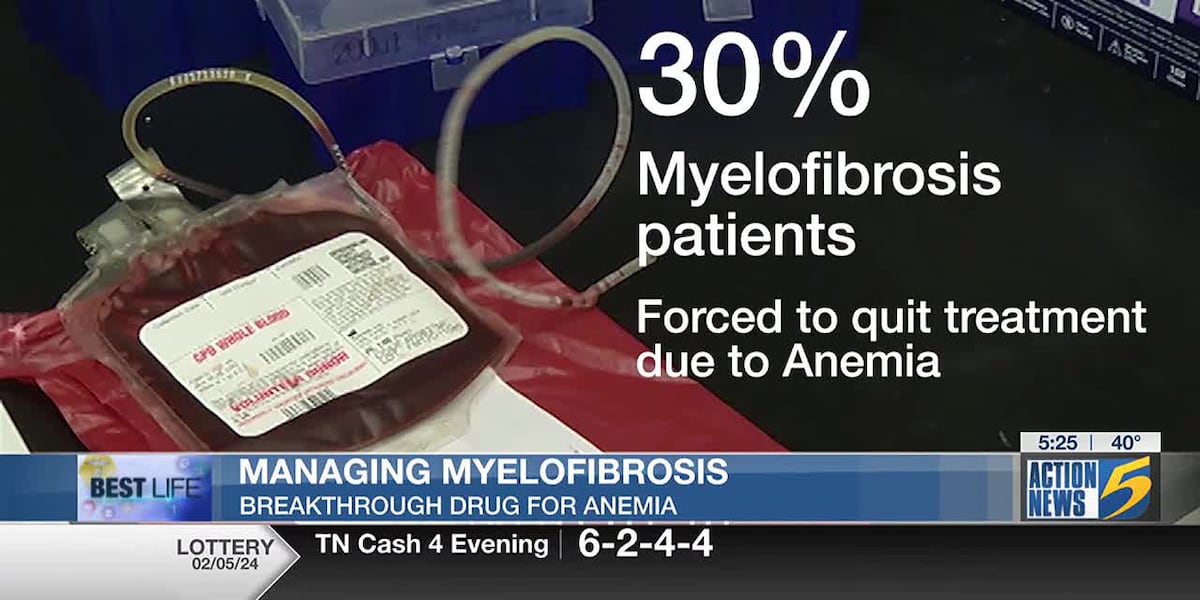
Myelofibrosis, a rare cancer affecting the blood’s ability to function correctly, impacts around 25,000 Americans. This disease is part of a group of cancers that collectively form about ten percent of all cancers and typically leads to severe anemia among other complications. A recent advancement in medical treatment has been achieved with the FDA’s approval of a new drug specifically designed to treat the anemia associated with myelofibrosis, potentially improving patient outcomes significantly.
Personal Stories Highlight Treatment Advances
The journey of Andrew Schorr, a myelofibrosis patient since 2011, underscores the struggles faced by individuals with this rare condition. After years of battling fatigue due to anemia—a common issue in myelofibrosis—Andrew found a glimmer of hope in the form of a new treatment option. This treatment, a daily medication named Ojjaara, targets specific pathways involved in the disease’s progression, offering patients a chance to manage their symptoms more effectively and maintain their lifestyle as much as possible.
Optimism Amidst Challenges
With the introduction of Ojjaara, patients like Andrew have a renewed sense of hope for managing their disease. His wife, Esther, shares this optimism, expressing joy at the prospect of spending many more years together. While the medication brings potential side effects, such as gastrointestinal discomfort and changes in platelet counts, the overall potential for improving quality of life makes it a pivotal development in the treatment of myelofibrosis.