In a groundbreaking achievement, researchers at the University of Cologne (UoC) have successfully synthesized artificial nucleotides, the fundamental components of DNA, in the laboratory, showcasing additional properties that could revolutionize their application as artificial nucleic acids for therapeutic purposes.
DNA, the carrier of genetic information in all living organisms, is comprised of four distinct nucleotides, each consisting of a sugar, a phosphate group, and one of four nucleobases: adenine, thymine, guanine, and cytosine.
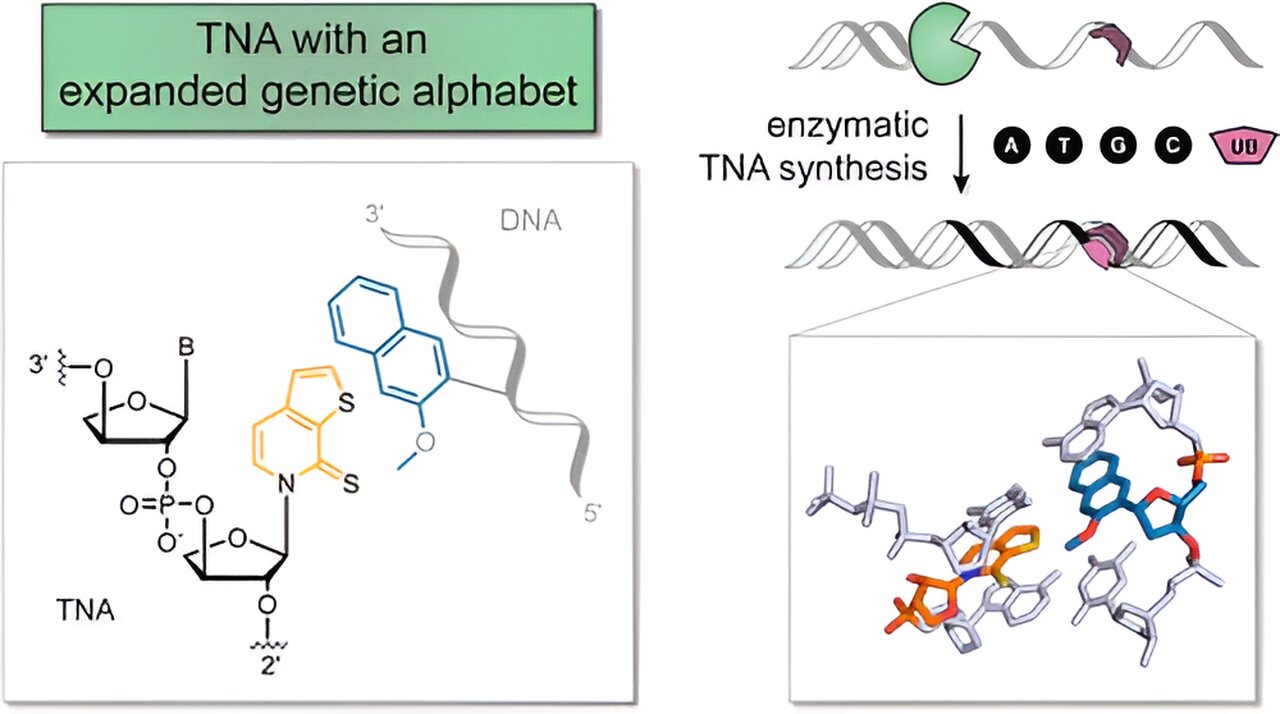
These nucleotides align repetitively to form the iconic double helix structure of DNA. Scientists at the UoC’s Department of Chemistry have demonstrated the remarkable versatility of altering this structure significantly within a laboratory setting. Their innovative work led to the creation of threofuranosyl nucleic acid (TNA) featuring a novel additional base pair.
This pioneering research marks a crucial advancement towards the development of fully synthetic nucleic acids endowed with enhanced chemical functionalities. The study titled “Expanding the Horizon of the Xeno Nucleic Acid Space: Threose Nucleic Acids with Increased Information Storage” was published in the Journal of the American Chemical Society.
Artificial nucleic acids exhibit structural deviations from their natural counterparts, impacting their stability and biological functions. Professor Dr. Stephanie Kath-Schorr highlighted the enhanced stability of threofuranosyl nucleic acid compared to DNA and RNA, underscoring its potential advantages for future therapeutic applications.
The study involved substituting the 5-carbon sugar deoxyribose, a key component of DNA, with a 4-carbon sugar and expanding the nucleobase repertoire from four to six. This modification renders TNA resistant to cellular degradation enzymes, addressing a significant challenge in nucleic acid-based therapeutics where synthetic RNA is rapidly degraded upon cellular entry, diminishing its efficacy.
The introduction of TNAs into cells incognito could prolong their therapeutic effects. Lead author Hannah Depmeier emphasized the unique binding capabilities of the incorporated unnatural base pair, offering novel targeting opportunities within the cell. Kath-Schorr envisioned leveraging this functionality for the development of aptamers—short DNA or RNA sequences—for precise modulation of cellular processes.
TNAs hold promise for targeted drug delivery to specific organs (targeted) and diagnostic applications, facilitating the detection of viral proteins or biomarkers.