In a pioneering experiment conducted in the early 1950s, a scientist endeavored to replicate the conditions of early Earth within a test tube.
By combining a few basic ingredients believed to have existed in the atmosphere and oceans of the young planet, Stanley Miller created interconnected flasks, applied heat, and subjected them to electrical discharge to mimic lightning. The outcome was remarkable: from this primordial mixture, amino acids—the fundamental building blocks of life—emerged.
This groundbreaking discovery sparked a scientific pursuit in the fields of chemistry and biology to develop experiments that could shed light on one of humanity’s most significant scientific inquiries: the origins of life itself.
Recently, researchers at Ludwig Maximilian University of Munich have progressed further by demonstrating how more intricate molecules essential for life could have been synthesized from the fundamental components present on early Earth.
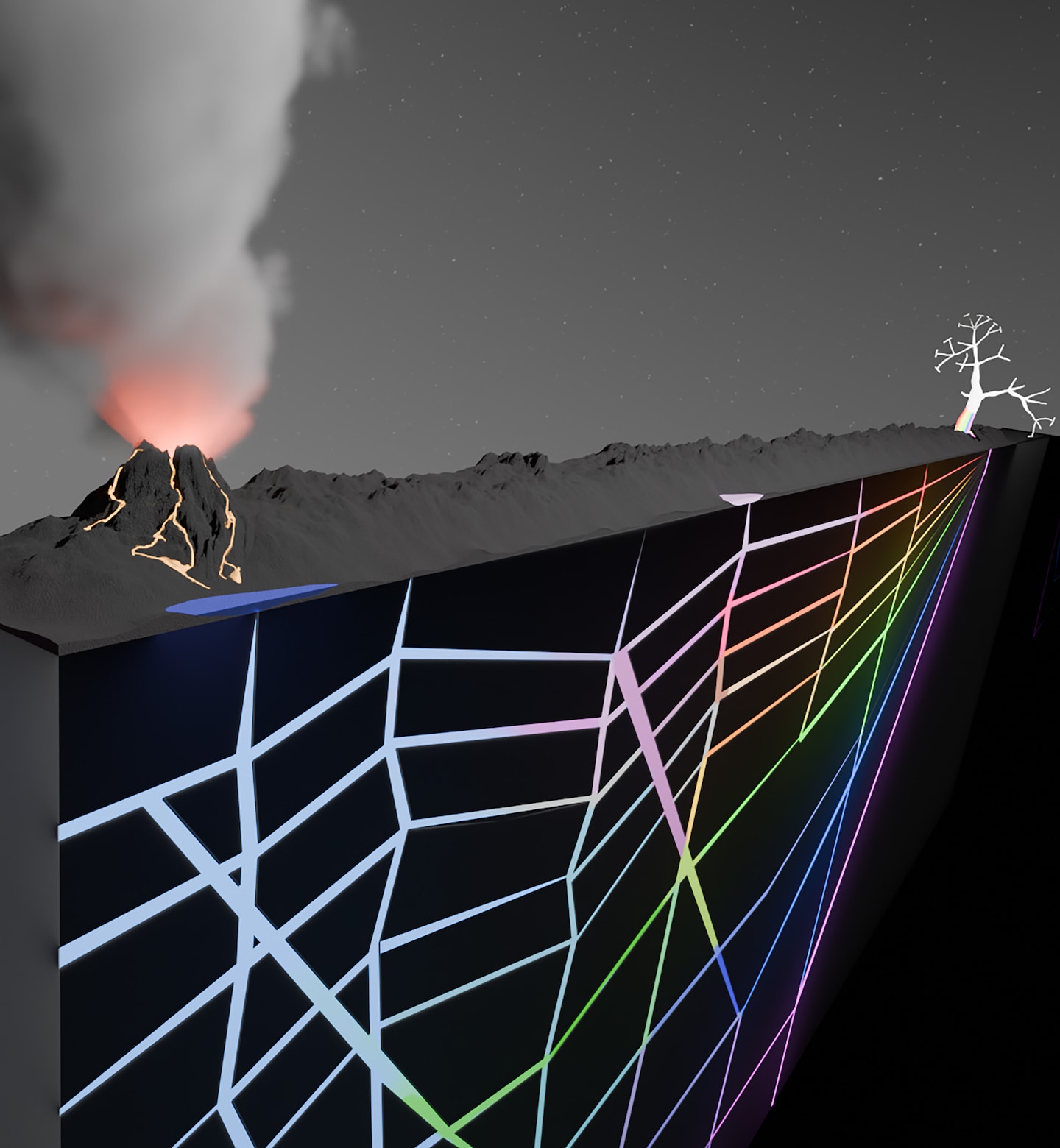
In their published study, the scientists transitioned from traditional test tubes to intricate networks of branching cracks resembling natural formations in rocks. By circulating water and essential chemical building blocks through these cracks and applying heat—simulating conditions akin to hydrothermal vents in oceans or porous rocks near geothermal pools—they observed a fascinating phenomenon. The heat distribution across these geological networks effectively organized and filtered molecules, facilitating the formation of longer chains known as biopolymers, crucial for life’s emergence.
Matthew Pasek, a geoscience professor at the University of South Florida not involved in the research, lauded the findings, stating, “It’s a fantastic demonstration that simple physical processes can work to do this stuff.”
The complexity of the question regarding life’s inception transcends disciplinary boundaries, engaging chemists, biologists, astrophysicists, and geologists alike in the quest for answers.
Christof Mast, a biophysicist at Ludwig Maximilian University of Munich, sought to bridge these disciplinary boundaries by designing an experimental setup that closely resembled the conditions conducive to the “prebiotic chemistry” responsible for life’s genesis.
The challenge lies in replicating the highly dilute and uncontrolled environment of the prebiotic Earth, where various reactions occurred in a chaotic manner. To tackle this, the researchers carved interconnected crack networks into a Teflon-like material and subjected it to controlled heat flux between sapphire plates, mimicking the heat flow on early Earth. By observing the flow of water and basic chemical components through these crack networks, they could unravel the mechanisms behind the synthesis of crucial building blocks for life.
The experimental results underscored the significance of heat gradients in enhancing the production of biopolymers from basic components, a pivotal step towards the formation of life. This innovative approach sheds light on the challenges early Earth faced in generating sufficient building blocks for life amidst a chaotic environment.
While the experiment cannot definitively pinpoint the exact location where life originated—whether in surface ponds or hydrothermal vents deep in the ocean—it provides valuable insights into early Earth’s geochemical processes. The study’s implications extend to exploring various geological settings and the potential ubiquity of heat flux across rocks on early Earth.
As researchers delve deeper into understanding early chemistry on our planet, the study serves as a testament to the intricate interplay of geological conditions in shaping the origins of life. The experimental approach adopted in this research, while not the sole mechanism, offers a geologically plausible and ingenious perspective, emphasizing the need for more experimental investigations to unravel the geochemical context of life’s emergence.